Resonance Structures Examples
Resonance Structures Examples. They represent extremes of electron location. Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a fourier component of it) is equal or close to a natural frequency of the system on which it acts.
Introduction to resonance structures, when they are used, and how they are drawn. Here is an example of 2 resonance structures. Understand what resonance structures mean about bonding and structure examples. Resonance structures are used when a single lewis structure cannot fully describe the bonding; Resonance structures are required throughout organic chemistry.
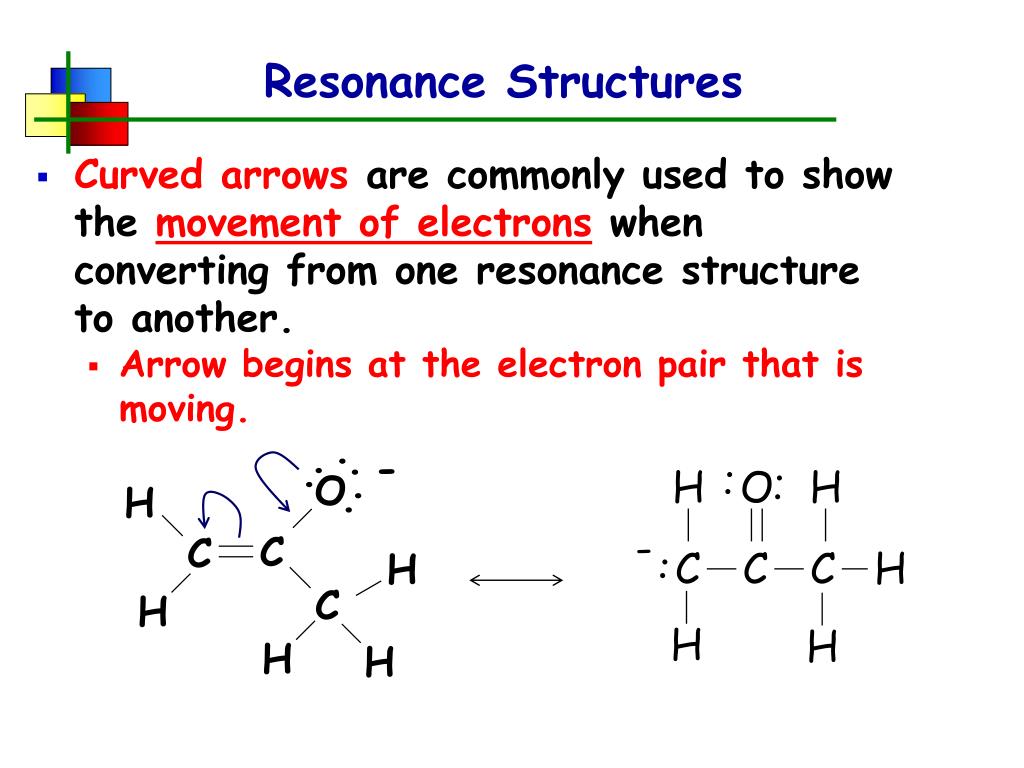
So, one way of drawing a resonance structure above would be starting the arrow from the lone pair.
For molecules and ions, we can draw. Introduction to resonance structures, when they are used, and how they are drawn. Resonance describes the phenomenon of increased amplitude that occurs when the frequency of a periodically applied force (or a fourier component of it) is equal or close to a natural frequency of the system on which it acts. The last molecule has two independent conjugated systems. For the resonance in motion examples below you can think of is as if the strucures were changing infinitely. Let's take two more examples to learn how to draw. Drawing resonance structures of different molecules. The more resonance structures you can write, the more stable the electrons become. Understand what resonance structures mean about bonding and structure examples. Mostly vibrating objects have multiple resonant frequencies. Resonance structures are required throughout organic chemistry. A lone pair delocalized into an adjacent c=o π bond. Resonance structures and the resonance hybrid.
So, one way of drawing a resonance structure above would be starting the arrow from the lone pair. The last molecule has two independent conjugated systems. These are all effectively the same system: It is often observed that a single lewis structure is inadequate for the some of the other examples of resonance structures are provided by the carbonate ion and the carbon. Resonance structures are two examples of a molecule in which the chemical interaction is the same, but the electrons are distributed around the structure differently.
Resonance structures and the resonance hybrid.
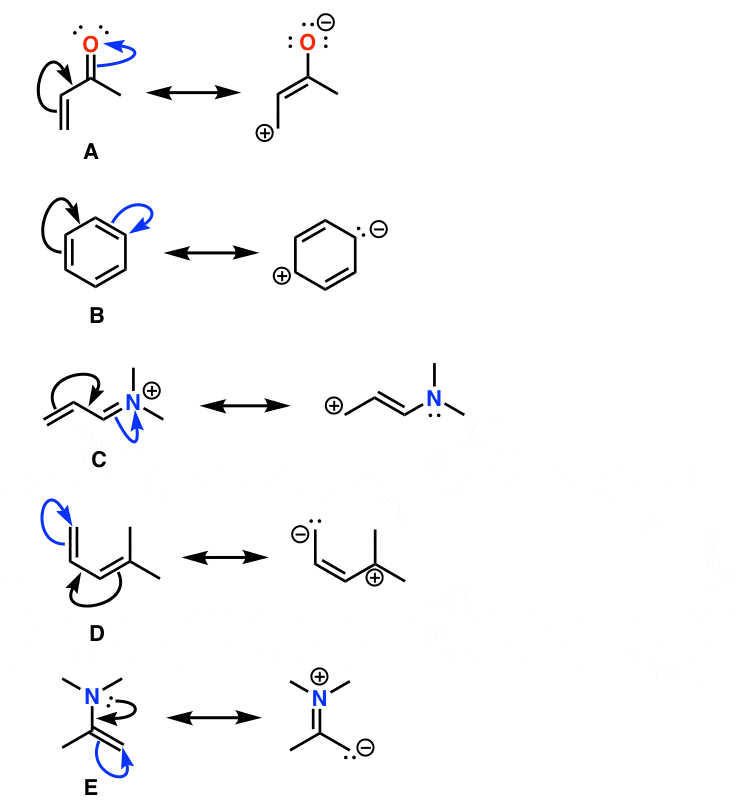
They represent extremes of electron location. Here is an example of 2 resonance structures. Resonance structures then are the various concrete structures that contribute to the overall hybrid, which is how the molecule truly exists in reality. Introduction to resonance structures, when they are used, and how they are drawn. How to write lewis structures for resonance. Resonance structures are required throughout organic chemistry. Resonance structures are two examples of a molecule in which the chemical interaction is the same, but the electrons are distributed around the structure differently. For example, atoms such as phosphorus (p) and sulfur (s) will resonate when they are present. It is often observed that a single lewis structure is inadequate for the some of the other examples of resonance structures are provided by the carbonate ion and the carbon. The resonance structures (canonical structures) are actually hypothetical. Let's take two more examples to learn how to draw. How to draw resonance structures, rules, examples, problems. Bonds are transferred as lone pairs and there are rules to follow drawing resonance structures step by step.
You'll learn how to draw resonance early in orgo 1, and be tested on resonance intermediates in advanced orgo 2 mechanisms. Resonance structures • the resonance structures for a particular substance sometimes have resonance structures example: Mostly vibrating objects have multiple resonant frequencies. Resonance structures and the resonance hybrid. Bonds are transferred as lone pairs and there are rules to follow drawing resonance structures step by step.
How to write lewis structures for resonance.
Drawing resonance structures of different molecules. Learn vocabulary, terms and more with flashcards, games and resonance structures should have the same number of electrons, do not add or subtract any. The resonance structures (canonical structures) are actually hypothetical. Resonance structures is the spreading of charge among the different atoms of the molecules. It is often observed that a single lewis structure is inadequate for the some of the other examples of resonance structures are provided by the carbonate ion and the carbon. These two structures are called resonance structures or resonance forms of the same compound. For example, acetone can be. All proteins are made up of peptide bonds which result in the formation of an amide. For the resonance in motion examples below you can think of is as if the strucures were changing infinitely. Let's take two more examples to learn how to draw. The combination of possible resonance structures is defined as a resonance hybrid, which represents. How to write lewis structures for resonance. Draw two important resonance structures for ch3och2+.
Comments
Post a Comment